

The vaccine is approved under the name Spikevax for people age 18 and older.

#Coronavirus vaccine after effects trial#
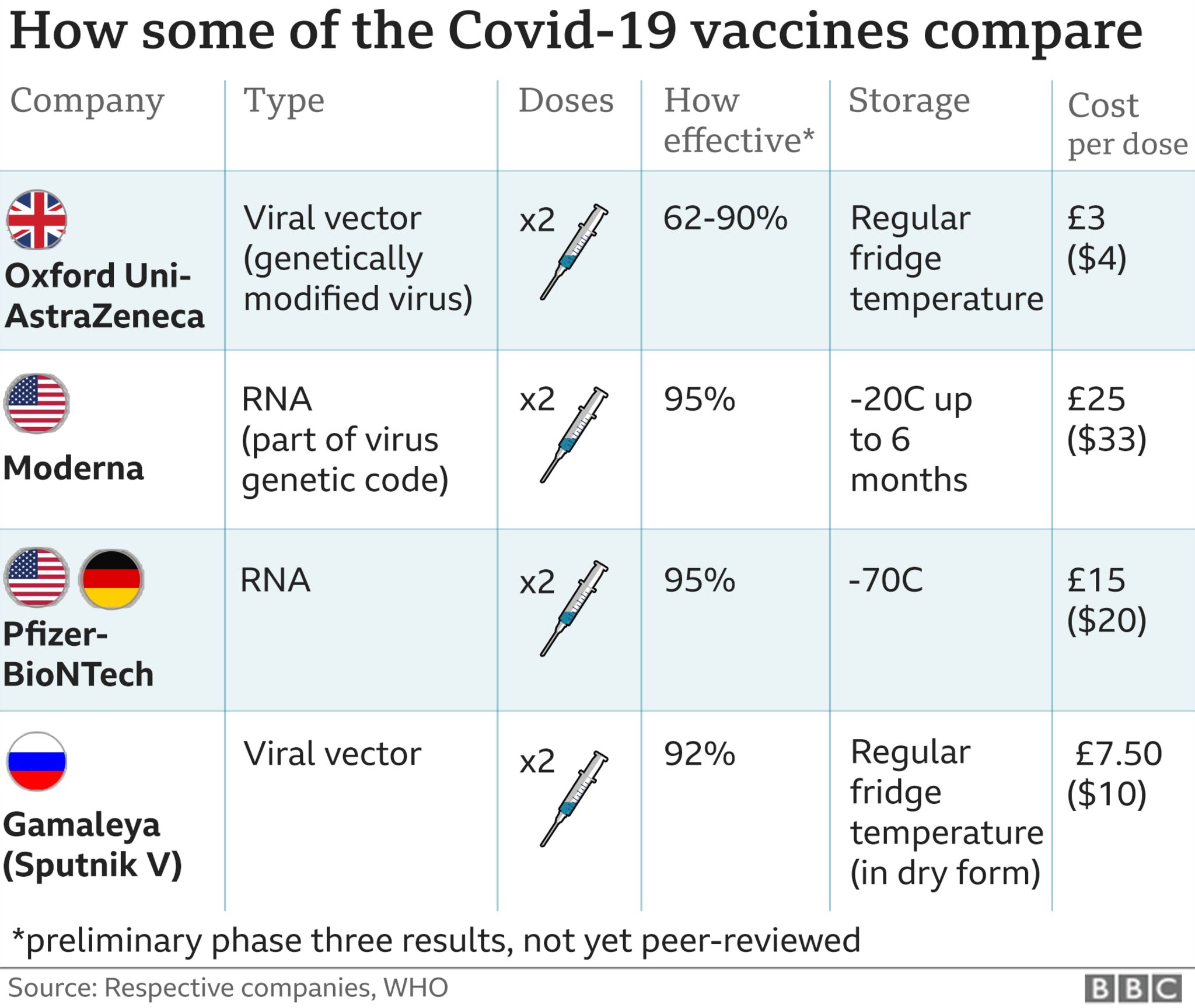
Based on clinical trial data the vaccine effect was predicted for younger people. In December 2020, the Moderna COVID-19 vaccine was found to be both safe and about 93% effective in preventing infection among study volunteers, all age 18 or older.īased on the comparison between people who got COVID-19 in the placebo group, the Moderna COVID-19 vaccine was 98% effective at preventing serious COVID-19 illness. This vaccine also was first tested against the original strain of the virus that causes COVID-19.
#Coronavirus vaccine after effects series#
The number of shots in this vaccination series varies based on a person's age and COVID-19 vaccination history. It is authorized for people age 6 months and older. The Pfizer-BioNTech vaccine is approved under the name Comirnaty for people age 12 and older. This data helped predict how well the vaccines would work for younger people. In December 2020, the Pfizer-BioNTech COVID-19 vaccine 2-dose series was found to be both safe and 91% to 95% effective in preventing COVID-19 infection in people age 18 and older. This vaccine was first tested against the original strain of the virus that causes COVID-19, which began spreading at the end of 2019. Vaccines with FDA emergency use authorization or approval include: But the data still had to show that the vaccines were safe and effective. As many work shifts, that naturally spaces out the process.So the FDA first gave emergency use authorization to COVID-19 vaccines based on less data than is typically required. In Britain, home to the AstraZeneca vaccine developed at Oxford University, the policy has been to make vaccinations readily available to hospital staff. The United States is administering shots from Pfizer/BioNTech and Moderna. hospitals and other organisations with front-line staff adopted a similar strategy when the country’s vaccination programme started in December. During this period a total of 10,000 people received the shot nationwide. The agency put out the advice after receiving 149 alerts of often strong flu-like side-effects from the AstraZeneca vaccine. It also issued guidance to stagger vaccinations of front-line staff working together in teams to minimise the risk of disruption to operations. 11 that such side-effects were “known and described” but should be subject to surveillance with regard to their intensity. People receiving the vaccine are closely monitored through routine pharmacovigilance activities, the Anglo-Swedish drug maker said, adding that it was continuing to keep a close eye on the situation.įollowing similar reports from other hospitals, the French medicines safety agency said on Feb. The other shots approved in Europe, developed by Pfizer and Moderna, have been linked to similar temporary side-effects, including fever and fatigue.īut with the AstraZeneca shot the latest to be rolled out, health authorities in France have issued guidance to stagger giving the shot, two regions in Sweden paused vaccinations, and in Germany some essential workers are refusing it.Ī spokesman for AstraZeneca said: “Currently, the reactions reported are as we would expect based on the evidence gathered from our clinical trial programme.” Such symptoms, as reported in clinical trials for the AstraZeneca shot, can include a high temperature or headache and are a normal sign that the body is generating an immune response. FILE PHOTO: A nurse administers the Oxford-AstraZeneca COVID-19 vaccine to a member of the medical staff at a coronavirus disease (COVID-19) vaccination center in La Baule, France, February 17, 2021.


 0 kommentar(er)
0 kommentar(er)
